With what you have learnt in the previous modules, l hope that you now have a little understanding of the need for adequate management of water – perhaps not for wastewater now; that will be treated later.
This is better summarized by the statement that the water sources are not always clean, and treating water to improve smell, taste, clarity, or to remove disease-causing pathogens in one form or another is often desired.
It is therefore the aim of this unit to take you into some water treatment issues that will provoke your thoughts. Meanwhile, it is very desirable that you begin by reviewing the history of drinking water treatment.
Guidelines and Standards for Water Quality
Typically, water quality is assessed by comparisons of water samples to water quality guidelines or standards. These guidelines and standards provide for the protection of human health, by ensuring that clean and safe water is available for human consumption.
There is a distinction between the two terms, guidelines and standards. The World Health Organisation’s (WHO) Water Quality Guidelines provide international norms on water quality and human health that are used as the basis for regulation and standard setting, in developing and developed countries world-wide.
These guidelines are adopted by many countries as national guidelines to follow, even if they are not necessarily enforceable by law.
In contrast, drinking water quality standards are primarily set by nation states and can be enforceable by law.
For example, the Environmental Protection Agency (EPA) of the United States of America has two sets of standards: the Primary Standards, that directly link human safety to drinking water and are enforceable by law, and the Secondary Standards, that relate to cosmetic and aesthetic effects and are not legally required.
Another example of binding standards is the Water Framework Directive set out by the European Union. Under Council Directive 98/83/EU, the Union provides Drinking Water parameters which include an obligation for EU member countries to inform the consumer on drinking water quality and measures that they can take to comply with the requirements of the Water Directive. EU members have agreed to comply with these parameters.
Many countries set drinking water quality guidelines based on the WHO guidelines but may modify these based on what is achievable in-country. For example, the financial requirements and infrastructure needed to monitor and assess drinking water quality can be limiting in some developing countries. For these and other reasons, the guidelines may also vary between rural areas and urban centres within a country.
Water Treatment Technology
Water treatment is used in many commercial operations, including food services, laundries, laboratories, pharmacies, and car washes. The type of treatment depends upon the application and the required water purity.
Treatment ranges from simple cartridge filtration to sophisticated systems that produce extremely pure water. For example, ice machines often have cartridge sediment and carbon filters installed on the make- up water, such that the ice is free of particles and chlorine taste.
Read Also : Issues in Wastewater Reuse and Recycling, and the Future of Water Reuse
Some laboratories and the pharmaceutical and electronics industries, however, require “ultrapure water,” which has had all but a few parts per billion of minerals, organics, and other substances removed through a train of treatment, including filtration, carbon filtration, softening, reverse osmosis, and strong acid/base ion exchange, followed by microfiltration and ultraviolet-light disinfection. Table 6 compares various treatments found in commercial operations.
Table 6: Different Water Treatment Options
| Treatment Process | |||||||
| Sediment Filtration | Carbon Filtration | Softening and Ion Exchange | Membrane Process | Distillation | Disinfection | Other Treatment Processes | |
| All food service | X | X | X | X | X | ||
| All laundry & dry cleaning | X | X | |||||
| Hospital & Laboratory | X | X | X | X | X | X | X |
| Car Wash | X | X | X | ||||
| Beverage Manufacturing | X | X | X | X | X | ||
| Metal Plating | X | X | X | X | X | X | |
| Cooling Tower & Boiler | X | X | X | X | X | ||
| Pool, Spa & Water Feature | X | X | |||||
| Office & Non – Process | X | X | X | X | X |
Each treatment technology offers unique opportunities for water conservation, as described below:
Sediment filtrationis one of the most common treatment techniques. Swimming pools, water feeds to commercial ice machines, cooling-tower side-streams, drinking- water and water- using medical equipment are but a few examples where sediment filters are found (e.g. Figure 5).
They remove particles down to a few microns in size. The two basic designs use disposable cartridges or granular filter media.
By their nature, cartridge filters are usually not designed for very large flows. Sample uses include pre-filters for ice machines, smaller medical equipment, and smaller swimming pools and spas.
Filter material varies from tightly wound fibers to ceramics, fused powdered-metals, or other materials. Such filters are left in place until the sediment buildup causes a predetermined increased pressure drop across the filter, at which time the filter is replaced, backwashed, or removed and cleaned for reuse.
The second type of sediment filter is often found where larger volumes of water must be processed or higher levels of sediment must be removed. These include granular media such as sand, coated media (cellulose, and perlite), and mixed-bed filters. All of these must be backwashed.
The backwash water is generally discharged to the sanitary sewer. In some larger applications, however, the sediment can be allowed to settle out and the clarified water can be reintroduced at the head of the filtration process. Common applications include swimming pools, industrial water treatment, and side-stream filtration for cooling towers.

Carbon filtrationremoves chlorine, taste, odour, and a variety of organic and heavy-metal compounds from water by adsorption.
Activated carbon, which has an enormous surface area per unit volume, attaches to the unwanted materials and holds them on its surfaces.
Restaurants and food service providers for hospitals and other institutional operations often use activated carbon for drinking water and ice-machine feed water. It is also used in the beverage industry for taste and odour control.
Activated carbon is also used to remove pollutants in the metal-finishing industry and other operations where pretreatment to remove metals or organics is needed.
These systems can employ either disposable cartridges or packed columns, where the activated carbon can be removed and sent for recharge. With both cartridge and packed-column systems, water simply pass through the carbon medium until its adsorptive capacity is used up.
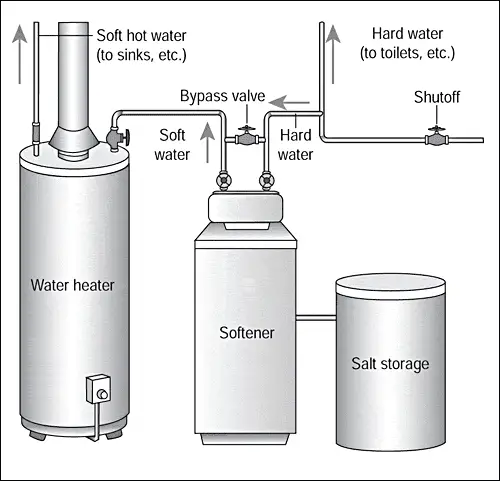
Water softeningemploys zeolites or ion exchange resins, where calcium and magnesium ions are exchanged for sodium or potassium ion. Softening removes hardness to control scale, improves water for washing, and prevents “hard water” spots.
Recharge is done with a salt solution containing sodium or potassium cations, the most common being sodium chloride (table salt).
Water is used in the recharging process to make up the brine solutions and to purge the softener of brine prior to being returned to service.
All softener systems should be equipped with controllers that are activated based upon the volume treated, not on timers. (e.g. Figure 6) They should either be adjusted for the hardness of the water supply or be equipped with a hardness controller that actually measures the hardness and volume treated, if the hardness of the feed water varies.
Softeners are commonly found where hardness interferes with water use or where scale formed by hard water could be detrimental.
Laundries, car wash, boiler feed-water, laboratory water, hot-water systems for restaurants and food-service establishments, and metal-plating operations commonly employ softening.
It is used occasionally for cooling-tower feed-water or in a process called side-stream softening, which helps extend the usefulness of cooling-tower water. Deionization also employs exchange resins, but it is different from softening.
Strong acid/base ion-exchange resins, known as deionization resins, are used to produce extremely pure water for laboratory analysis, kidney dialysis, and feed-water for a number of industrial processes.
Water use is similar to that for recharging softening systems, but the discharge water can be much more corrosive.
Controls should be based upon the chemistry of the feed water and volume treated, not on timers. Ion-removal systems operate similarly to ion-exchange systems and have similar water-use patterns. Ion exchange resins can also remove a variety of ionic contaminants, such as arsenic or fluoride.
Membrane processesinclude several water-treatment methods. A membrane, usually composed of a polymer material, is used to remove contaminates. All membrane processes have three things in common:
There is a feed stream, a retentate or waste stream, and products called permeate. The type of membrane process used depends upon the size or type of contaminant one wishes to remove.
Microfiltration employs membranes that remove particles of 0.1 to 10 microns in size or larger. It is used in municipal water treatment to remove bacterial and Giardialambliacysts, and Cryptosporidium oocysts. Water is forced through the membrane until the pressure drop reaches a set point.
The filter is then backwashed. The membranes also require periodic chemical cleaning. Both the backwash and cleaning processes use water. Retentate or waste volumes are usually a small percentage of the total feed volume. The retentate is often reticulated and only a small stream of “bleed water” is discharged as wastewater. Some ceramic filters can also filter in this range.
Ultra filtration operates at higher pressures than microfiltration and removes materials that are much smaller, including viruses and proteins. It is often used to separate milk and whey. These filters must be backwashed and cleaned in a manner similar to microfiltration membranes.
Nanofiltration membranes have pore sizes midway between those of ultra filtration and reverse osmosis. Nanofilters are often referred to as “softening” filters, since they are effective in removing multivalent cations such as calcium and magnesium.
Reverse osmosis (RO) removes salts from a water stream (Figure 7). It finds use wherever very pure water is needed, such as laboratories, medical uses including kidney dialysis, metal plating, boiler feed-water, and a number of related applications.
Read Also : Types of Wastewater and Motivational Factors for Recycling/Reuse
Typically, RO will reject 90 to 95 percent of the salts. RO is also used before strong acid/base deionization for the production of ultrapure water for laboratory, pharmaceutical, and microelectronics manufacturing operations.
Distillation, a process once in common use to make water for laboratory applications, is still found in many laboratories. Electric or gas stills are used.
Production quantity depends upon the size of the still. Smaller stills often use once-through condenser water and can waste huge volumes of water to produce a single gallon of distillate. Small and medium size stills use air to cool the coils and have no discharge.
These are the most water- efficient stills. Some larger stills have reject streams to prevent scale buildup. These typically dump 15 to 25 percent of the water entering the still.
Disinfection and other technologiescan consume small amounts of water, if chemicals are fed in a liquid or slurry form.
Chemical disinfection technologies include use of chlorine compounds, ozone, and hydrogen peroxide, as well as pH control with acids and bases and the addition of antiscalants and sequestrates such as sodium hexameter phosphate.
Ultraviolet light, heat, and extreme mechanical sheer are among other technologies in use. It is important to examine disinfection requirements. Ultraviolet light, heat, and mechanical sheer processes do not use water.
Other processes use water to make up the solutions, but this becomes part of the product water and is not lost. However, cleaning chemical storage areas does consume water.
The potential for water savings by choosing among disinfection technologies is not great; however, the potential to waste water in cleaning the equipment and storage vessels is a concern which use of waterless methods can lessen.
In conclusion, contaminated water is not desirable for consumption but could be treated to make it consumable. A number of approaches have been discussed.

