What, then, must be removed from public water supplies, and what other chemicals are added to the water?
How do public water facilities treat our water to make it safe for us to drink and appropriate for other human uses?
There are six major steps in the treatment of our water: screening, sedimentation, precipitation, filtration, adsorption, and disinfection. Some of these steps, such as precipitation, involve chemical reactions among the aqueous species dissolved in the water; others, such as screening, involve only separation of particles on the basis of physical characteristics like size.
Many of these steps depend on one another. For instance, precipitation generates solids in the water from particles that had been dissolved; these solids must then be removed through sedimentation or filtration.
We shall discuss each of the six steps in water treatment below, and then present a schematic showing how the steps work together to produce clean, usable freshwater.
Surface water (water from lakes and rivers) often has large debris, such as sticks, leaves, fish, and trash, floating in it. These objects can clog the water-treatment system and must be removed before the water enters the treatment plant.
Read Also : Efficiency and Maintenance of Waste-water Treatment Units
Treatment facilities that use surface water have large screens covering the site of water intake. The debris is too large to pass through the holes in the screens. Thus, as the water enters the plant, the large debris is removed.
1. Screening
The screens must be cleaned periodically to remove any objects that have become stuck, so that they do not clog the screen and impede water flow into the plant.
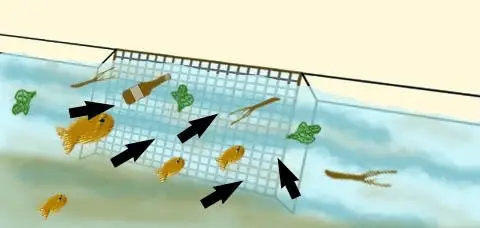
Figure 4: screening of large objects in surface waterwww.researchgate.net
This drawing shows some of the large objects in surface water that are removed as the water pass through a screen into the water-treatment facility. The large black arrows show the direction of water flow through the screen.
2. Sedimentation
Other suspended (insoluble) particles, such as sand and dirt, are small enough to pass easily through the screens. These particles must be removed from the water by another process known as sedimentation.
When water is allowed to sit, heavy suspended particles (e.g., sand) will settle to the bottom over time because they are denser than water. The water, now free of the suspended impurities, can be collected from the top without disturbing the layer of sediment at the bottom (which is eventually discarded).
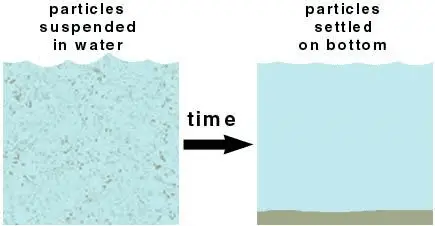
Figure 5: suspended and settled particles in waterwww.researchgate.net
Particles that are insoluble in water may be suspended in the water, particularly if the water is turbulent (stirred up). If the particles are heavy enough, they will settle to the bottom when the water is allowed to sit still over time.
Sometimes the insoluble particles are too small to settle out quickly enough to use sedimentation alone. Two processes, known as flocculation and coagulation, are used to create larger particles that will settle quickly to the bottom.
In flocculation, small particles with non-rigid surfaces are made to agglomerate by mixing the water (and thus bringing the particles into contact with one another so that the surfaces can become stuck together).
When the agglomeration of the particles gets large enough, the aggregate can settle in still water by sedimentation. Other suspended particles do not agglomerate well by flocculation. To remove these particles from the water, coagulation must be used.
Coagulation is the process of gathering particles into a cluster or clot, often achieved by the addition of special chemicals known as coagulants. The most common coagulant used in water- treatment facilities is aluminum sulfate (alum, Al2 (SO4)3).
Other Al and Fe salts, including poly-aluminum chloride, ferric chloride, and ferric sulfate, may be used as well. These salts react with ions naturally found in the water to produce a solid precipitate (Equation below).
As this precipitate forms, other particles are caught in the solid, forming a mass that will settle to the bottom via sedimentation as shown in the diagram below.
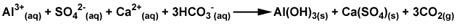
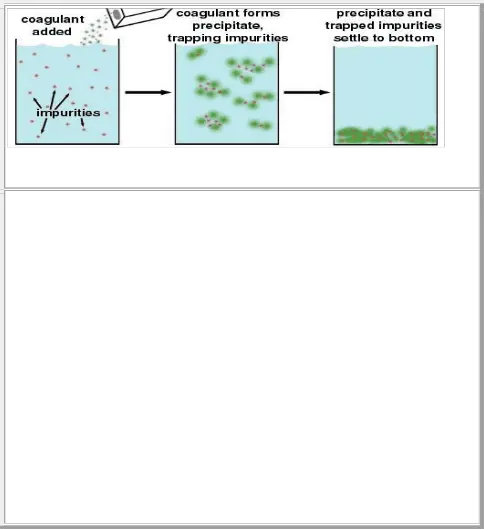
Figure 6: Coagulation of suspended particles in water treatment plant.
www.researchgate.net
When coagulants such as Al2(SO4)3 are added to the water supply, they form solid precipitates (green), as shown in Equation 2, above. These precipitates catch other impurities (red) in the water, forming a solid mass containing the precipitate formed by coagulation and the trapped impurities. This mass will settle to the bottom by sedimentation, and the water (with the trapped impurities now removed) can be drained off from the top.
3. Precipitation
The steps in the water-treatment process described above are used to remove insoluble particles from the water supply. But recall from above (“Species (Other than H2O) Contained in Water”) that water also contains many molecules and ions in solution.
Many of the ions in solution can be removed by precipitation: reacting the ions (to be removed) with other ions to produce insoluble solids that can be removed by sedimentation.
Read Also : Meaning and Concepts of Design of Waste-water Treatment Units
A typical precipitation reaction used to remove ions in water treatment follows the reaction shown in the equation below. This is the same reaction type that you performed in the Experiment when the reaction between ions from two aqueous solutions produced a solid precipitate.
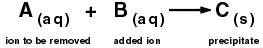
Two major classes of ions are typically removed via precipitation:
- Calcium (Ca2+) ions and magnesium (Mg2+) ions that have been leached from minerals in the ground cause the condition known as “waterhardness”. These ions do not pose any health threat, but they can engage in reactions that leave insoluble mineral deposits, such as scum rings on bathtubs and cooking vessels, or scale on industrial boilers, which decreases the boilers’ efficiency. These deposits can make hard water unsuitable for many uses.
- Iron (Fe2+) ions and manganese (Mn2+) ions can stain plumbing fixtures and laundered clothes. These ions may also promote the growth of certain bacteria, which give foul tastes and odors to the water.
Treating Water Hardness
The process of removing Ca2+ and Mg2+ from the water is known as watersoftening. Two minerals, lime (Ca (OH)2) and sodaash (Na2CO3), are typically used to soften public water supplies. (Incidentally, one important source of lime is
3 near St. Genevieve, Missouri.)
When lime is added to water, it dissolves to give three aqueous (solvated) ions: one Ca2+ ion and two OH– ions for each unit of Ca (OH)2. Likewise, soda ash dissolves to give two Na+ ions and one CO 2- ion for each unit of Na2CO3 that dissolves.
A number of reactions occur to generate the insoluble precipitates CaCO3(s) and Mg (OH) 2(s) from the Ca2+ and Mg2+ ions.
Removing Iron and Manganese
Two types of precipitation reactions may be used to remove Fe2+ and Mn2+ from water.
1. The most important of these reactions is oxidation. Using molecular oxygen (O2) or another oxidant such as potassium permanganate (KMnO4), Fe (II) is readily oxidized to Fe (III) in solution the equation below.
If the solution is alkaline (high pH, basic), the Fe (III) forms Fe (OH)3. As the concentration of Fe (OH)3 increases, the oxygens start to coordinate between multiple iron ions, and a lattice begins to form. (Recall the definition of a lattice from the discussion above on “The Solvation Process.”)
At some point in this lattice formation, the Fe (OH)3 starts to look like Fe2O3 (rust) and precipitates. Hence, by adding an oxidant to the water and raising the water’s pH at the water-treatment plant, an insoluble precipitate is formed. The insoluble rust can then be removed by sedimentation or filtration.
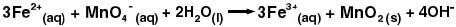
2. The water-softening agents described in the “Treating Water Hardness” section above can also help to make insoluble precipitates from Fe2+and Mn2+.
- Equation 4 shows how Mg2+ is removed using lime. However, this process introduces the new water-hardness problem of Ca2+ in the water.
4. Filtration
Often, the particles generated by the precipitation reactions described above are too small to settle efficiently by sedimentation. One strategy that is frequently employed to remove these solids is gravityfiltration.
In this process, water containing solid impurities (e.g., precipitates from water softening) is passed through a porous medium, typically layers of sand and gravel. The force of gravity is used to push the water through the medium. The small water molecules pass through the holes between sand and gravel pieces.
Read Also : Efficiency and Maintenance of Waste-water Treatment Units
Gravity filters at water-treatment plants have a pipe feeding into the under drain, the bottom layer where the clean water is collected. By adding water to the filter through this pipe, clean water can be forced upward through the filter to remove the solids that have collected in the filter. This process is used to clean the filter.
However, the solids (from precipitation) get stuck in the holes, and are thus retained in the porous medium. The water that passes through the bottom of the filter no longer contains those solid impurities.
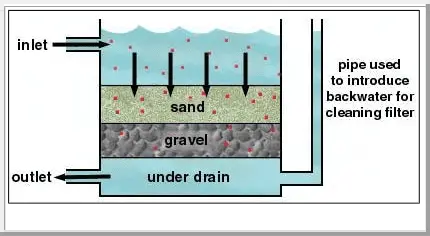
5. Adsorption
Dissolved organic compounds in water (e.g., atrazine, an herbicide, and industrial waste products) can pose a significant health threat, and may affect the taste and odor of drinking water. To remove them, the process of adsorption is used.
Adsorption is a process in which one substance is attached to the surface of another substance. Powderedactivatedcarbon (PAC), a finely ground charcoal, is used for this process. When PAC is added to the water, the organic compounds attach to the surface of the powder granules.
The granules of PAC have irregularly shaped surfaces, which gives PAC a very large surface area to attract organic compounds. It is estimated that 1 pound of PAC has a surface area of 100 acres! The carbon can then be removed by filtration, taking the unwanted organic compounds with it.
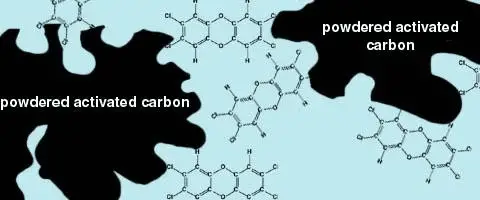
Figure:
This figure shows the irregular surface area of powdered activated carbon (PAC). Organic molecules, such as the dioxin (a toxic byproduct of chemical bleaching and a precursur to powerful pesticides) shown here, adhere to the surface of the PAC granules. When the PAC is removed by filtration, the adsorbed organic molecules are removed with the carbon.
6. Disinfection
In many water supplies, the most serious health threats are posed not by chemicals, but by infectious organisms (bacteria) in the water. Chlorine (Cl2) is a major disinfectant that is cheap and kills most of the serious disease-causing bacteria in the water. However, chlorine disinfection results in a wide variety of by-products.
One class of chlorination by-products, known as trihalomethanes (THM’s), are suspected carcinogens. Because of concern about these by-products in the water supply, chlorine is now kept to minimum levels, and other methods of disinfection are being used more frequently.
Chloramines form more stable disinfectants and pose less risk of harmful by-products, but cost more to use. Other methods focus on removing the organisms through coagulation, sedimentation, and improved filtration.
Addition of Other Chemicals to the Water Supply
Certainly a principal objective of the water-treatment process is to remove substances from water that are harmful, or that otherwise make the water unsuitable for human use. However, another important component of the process is the addition of chemicals that make the water better for human use.
For example, fluoride (F–) is routinely added to public water supplies to protect the teeth of those who drink the water. Cities that add appropriate amounts of fluoride to their drinking-water supplies have successfully reduced the incidence of cavities among the children who inhabit those cities.
Schematic of a Water-Treatment Plant
The processes of screening, sedimentation, precipitation, filtration, adsorption, and disinfection work together to remove the unwanted substances from our water supply, making it safe to drink and appropriate for other uses. Addition of other chemicals, such as fluoride, further enhance the quality of the water for drinking.
The figure below, depicts a flowchart showing how these processes work together. Once the water is treated, it is sent to storage chambers and then distributed to household consumers, businesses, and industries.
In conclusion, an understanding of chemistry is so important to the water-treatment process that water-treatment facilities hire many chemists to analyze the quality of the water and oversee its treatment. One of the most fundamental chemical principles in water treatment is solubility.
It is imperative to understand which contaminants are soluble (forming solutions) and which are insoluble (forming suspensions), in order to determine how they can be effectively removed. Insoluble contaminants can usually be removed by physical-separation processes, including screening, sedimentation, and filtration.
Read Also :Meaning and Concepts of Design of Waste-water Treatment Units
These physical processes may be aided by chemical processes, such as coagulation, that help to entrap the suspended particles. Soluble contaminants, on the other hand, must be removed by chemical methods that render them insoluble, so that they can then be removed by physical means, such as sedimentation and filtration. Furthermore, an understanding of solubility is essential in choosing reactants that will generate insoluble precipitates with the dissolved contaminants.
In summary, water is perhaps the most important nutrient in our diets. In fact, a human adult needs to drink approximately 2 liters (8 glasses) of water every day to replenish the water that is lost from the body through the skin, respiratory tract, and urine. But some water sources cannot safely be used to meet our requirement for drinking water. In fact, 99.7% of the Earth’s water supply is not usable by humans. This unusable water includes saltwater, ice, and water vapor in the atmosphere.

