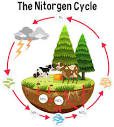
Living organisms require several nutrients either in trace or macro amounts for growth, maintenance, production and reproduction and Nitrogen one of those nutrients can be gotten from the Nitrogen cycle.
The atmosphere contains about 78% nitrogen though this is not directly usable by living organisms. Nutrient cycles describe the processes of inter-conversions that occur in nature.
These transformations can occur due to biological and non-biological processes. In this unit, we shall discuss the nutrient cycles as they occur in the aquatic environment.
The Processes of the Nitrogen Cycle
Many forms of nitrogen are present in the environment such as organic nitrogen, ammonium (NH4+), nitrite (NO2-), nitrate (NO3-), and nitrogen gas (N2).
Microorganisms are involved in many of the processes either to produce energy for their own use or to accumulate nitrogen for growth.
a. Nitrogen fixation
During nitrogen fixation, atmospheric nitrogen is converted to ammonium by lightening or by the activities of microorganisms. Free-living bacteria such as Cyanobacteria, Azobacter; or symbiotic bacteria (e.g. Rhizobium species) also fix nitrogen.
The bacteria change atmospheric nitrogen to ammonia and then to organic compounds. Rhizobium spp inhabit the root nodules of leguminous plants (e.g. peas or cowpea).
Read Also: An Overview of The Environment and It’s Types
The bacteria share the ammonia with the host plant in exchange for carbohydrate and accommodation.
Small amounts of nitrogen can be fixed by other natural activities such as forest fires and volcanic activities.
The nitrogen is converted to protein by the plant. Ammonia is also fixed industrially using Haber-Bosch process.
Under great pressure at 600˚C, with iron acting as catalyst, atmospheric nitrogen and hydrogen (from natural gas or petroleum) are combined to form ammonia (NH3) for producing fertiliser and explosives.
b. Assimilation
Plants absorb nitrogen from the soil as nitrate or ammonium ions.
Animals meet their requirements for nitrogen by eating plants.
The nitrate absorbed by plants is reduced to nitrite then ammonium ions before conversion to amino acids, nucleic acids and chlorophyll.
In plants that have a mutualistic relationship with Rhizobium spp, some nitrogen is assimilated in the form of ammonium ions directly from
the nodules.
Animals, fungi, and other heterotrophic organisms absorb nitrogen as amino acids, nucleotides and other small organic molecules.
c. Ammonification (mineralisation)
Ammonification or mineralisation involves the conversion of organic nitrogen from dead plants and animals or metabolic wastes from animals and plants back into ammonium ions (NH4+) by bacteria or fungi.
d. Nitrification
This is the conversion of ammonium to nitrate by nitrifying bacteria. Ammonium (NH4+) is oxidised to nitrite (NO2-) by Nitrosomonas species while Nitrobacteria species is responsible for oxidation of
nitrites to nitrates (NO3-).
It is important for the nitrites to be converted to nitrates because accumulated nitrites are toxic to plants. Ammonia and nitrite are toxic while nitrate is the least toxic.
However high nitrate concentration in drinking water causes methhemoglobinuria or blue baby syndrome. It may also add to
nutrient excess in receiving waters.
e. Denitrification
This is the conversion of oxidised forms (e.g. nitrates, nitrites) of nitrogen to the gaseous forms dinitrogen or to a lesser extent nitrous oxide gas (N2, N2O).
This process is carried out by denitrifying
bacteria such as Pseudomonas and Clostridium under anaerobic conditions (without oxygen) though these bacteria can also survive under aerobic conditions.
f. Nitrogen mineralisation
After nitrogen is incorporated into organic matter, it can be converted back into inorganic nitrogen by a process called nitrogen mineralisation or decay.
When organisms die, decomposers (bacteria and fungi) consume the organic matter during decomposition.
A significant amount of the nitrogen contained in the dead organism is converted to ammonium which can be used by plants or transformed into nitrate (NO3-) through nitrification.