Corrosion can occur on the outside of a pipe (due to corrosive soil) or on the inside of a pipe (due to corrosive water.) Either outside or inside a pipe, the creation of the corrosion cell can be through electrolysis, oxygen concentration cells, or through galvanic action.
1. Electrolysis
In electrolysis, a D.C. electric current enters a metal pipe and causes flow of electrons through the pipe and to the ground. The pipe, fueled by the electric current, becomes the anode while the soil becomes the cathode. The outside of the pipe corrodes, with the metal from the pipe plating out in the surrounding soil.
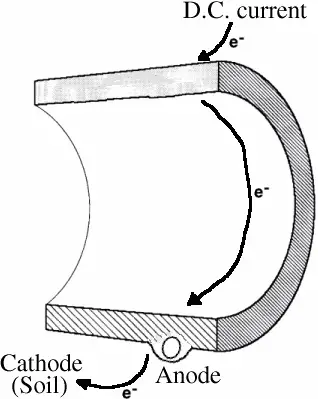
Electrolysis can occur when D.C. electric currents from nearby electric transit systems are grounded onto pipes. More commonly, the water and its constituents may set up a corrosion cell within the pipe.
These corrosion cells, known as oxygen concentration cells, result from varying oxygen concentration in the water. The portion of the pipe touching water with a low oxygen concentration becomes the anode while the part of the pipe in contact with a high oxygen concentration becomes the cathode.
Oxygen concentration cells are probably the primary cause of corrosion in the distribution system. They may occur at dead ends in the distribution system where water is stagnant and loses its dissolved oxygen. Alternatively, oxygen concentration cells may begin in annular spaces, which are ring-shaped spaces between two pipes or between a pipe and a pipe lining.
Oxygen concentration cells can also be caused by bits of dirt or bacteria. Both of these can become attached to the pipe walls, shielding the metal from dissolved oxygen in the water and setting up an anode.
Read Also: How Climatic Factors Affect Crop Production (Plant Growth)
2. Galvanic Corrosion
Metals themselves can also set up corrosion cells. When a pipe consists of only one type of metal, impurities in the pipe wall can develop into anodes and cathodes. Alternatively, when two dissimilar metals come into contact, galvanic corrosion will occur.
Galvanic corrosion is often set up in the distribution system in meter installations and at service connections and couplings.
Although inactive metals would make non-corrosive pipes, they are usually too expensive to use for the distribution system.
Galvanic series arrange the metal according to their tendency to corrode. At one end of the series are the metals which are less active and resist corrosion.
At the other end of the galvanic series are metals which are very active and have a high tendency to corrode. These metals can be used as sacrificial anodes in cathodic protection.
They should not be used for distribution system pipes. Most of the metals used in piping – iron and steel, are found in the middle of the galvanic series, and have some tendency to corrode.
The distance on the galvanic series between two metals will also influence the likelihood of galvanic corrosion when the two metals are placed in conjunction with each other.
For example, if aluminum is brought in contact with a steel pipe, the likelihood of corrosion is low since aluminum and steel are close together on the galvanic series.
However, if a stainless steel or a bronze fitting is used on an iron pipe, the likelihood of corrosion is much higher. When galvanic corrosion occurs, the more active metals always become the anodes. This means that they are corroded, and in extreme cases can begin to leak. The less active metal becomes the cathode and is not damaged
Read Also : Processes of Sludge Treatment

