Water, one of man most precious resources and an important component of the environment, is not well appreciated until reduced availability or quality or both threaten its use (Ohwo, 2010). Water is the most abundant compound in the biosphere.
It has a volume of about 1½ billion cubic kilometers. Of this quantity 97% is salt water, which is a major disadvantage to man. Only 3% is fresh water, of which about three quarter is locked up in the poles as ice and of course on the top of high mountains. Less than one percent is left in lakes and rivers for human use.
In order to appreciate water as a resource, we will examine its basic properties, which include the states of water, water as a universal solvent, a source of energy, its effect on climate, and structure of its molecule.
Structure of Water Molecule
The distance of Earth from the sun places it within a remarkable temperate zone compared with the other planets. This temperature location allows all states of water-ice, liquid and vapour, to occur naturally on earth.
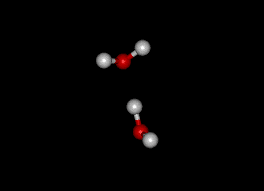
Two atoms of hydrogen and one of oxygen (H2O), which readily bond, compose a water molecule. The resulting molecule exhibits a unique stability, as a versatile solvent, and possesses extraordinary heat characteristics (Christopherson, 2007).
As the most common compound on Earth’s surface, water exhibits uncommon properties. Because of the nature of the hydrogen-oxygen bond, the hydrogen side of the water molecule has a positive charge and the oxygen side a negative charge; hence it dissolves so many other molecules and elements. Thus, because of its solvent activity, pure water is rare in nature (Christopherson, 2007).
Water molecules are attracted to each other because of their polarity. The positive (hydrogen) side of one water molecule is attracted to the negative (oxygen) side of another. The bonding between water molecules is called hydrogen bonding, it is important to note that without hydrogen bonding, water would be a gas at normal surface temperatures.
Different States of Water
Water can exist in three states – Solid (ice), liquid (water), and gas (water vapour). A change of state from solid to liquid, liquid to gas or solid to gas requires the input of heat energy called latent heat, which is drawn in from the surroundings and stored within the water molecules.
When the change goes the other way, from liquid to solid, gas to liquid, or gas to solid, this latent heat is released to the surroundings.
Water (Liquid Phase): As a liquid, water assumes the shape of its container and is a non-compressible fluid, quite different from solid, rigid ice. For ice to melt, heat energy must increase the motion of the water molecules to break some of the hydrogen bonds.
Despite the fact that there is no change in sensible temperature between ice at 00C and water at 00C, 80 calories of heat must be added for the phase change of 1 gram of ice to 1 gram of water.
Read Also : The Social Environment Structure of the Environment
This heat is called latent heat because it is stored within the water and is liberated with a phase reversal when a gram of water freezes. These 80 calories are the latent heat of melting and of freezing.
A calorie is the amount of energy required to raise the temperature of 1 gram of water (at 150C). To raise the temperature of 1 gram of water from freezing at 00C (320f) to boiling at 1000C (2120f), we must add 100 additional calories, gaining an increase of 10C (1.80f) for each calorie added (Christopherson, 2007).
Water Vapour (Gas Phase): Water vapour is an invisible and compressible gas in which each molecule moves independently. To accomplish the phase change from liquid to vapour at boiling temperature, under normal sea-level pressure, 540 calories must be added to 1 gram of boiling water. Those calories are the latent heat of vaporization. When water vapour condenses to a liquid, each gram gives up its hidden 540 calories as the latent heat of condensation.
In summary, taking 1g of ice at 00C and changing its phase to water, then to water vapour at 1000C – from a solid to a liquid, to a gas – absorbs 720 calories (80 cal + 100 cal + 540 cal). Reversing the process, or changing the phase of 1g of water vapour at 1000C to water, then to ice at 00C, liberates 720 calories into the surrounding environment.
Ice (Solid Phase): Among common compounds and molecules, water is the only one whose solid form is lighter than its liquid form (it expands by about 8% when it freezes, becoming less dense), which is why ice floats. If ice were heavier than liquid water, it would sink to the bottom of water bodies; the biosphere would be vastly different from what it is, and life, if it existed at all, would be greatly altered.
If bodies of water froze from the bottom up, they would freeze solid, killing all life in the water. Cells of living organisms are mostly water. As water freezes and expands, cell membranes and walls rupture. Finally, water is transparent to sunlight, allowing photosynthetic organisms to live below the surface (Botkin and Keller, 1998).
Note the molecular arrangement in each state and the terms that describe the changes from one phase to another as energy is either absorbed or released. Also note how the polarity of water molecules bonds them to one another, loosely in the liquid state and firmly in the solid state.
The plus and minus symbols on the phase changes denote whether heat energy is absorbed (+) or liberated (-) during the phase change.