The essence of this article is to have a review or summary of all of them, highlighting the major or essential points in each of them and bringing in some latent details not yet shown, all aimed at making sure that at the end of the day, no student will be left in the dark concerning sewage and waste-water treatment processes.
You should know that more than one treatment is needed to achieve the desired change in quality of waste-water. Thus, it is a chain processes operating in sequence. In some cases, treatment processes do not destroy the impurity but simply concentrate them in the form of sludge or effluent stream.
Selection of proper method based on the characteristics of waste-water is the key to solving treatment problems.
Nature of Impurities
It should be known that rainwater, surface water, groundwater, seawater and waste-water can be considered as the different forms of some basic material, varying in form and amount of impurities. These impurities decide the level of treatment that should be provided for any of its use and or disposal.
While municipal water has many types of impurities like floating and large suspended solids (paper, rags, plastics, and grit), dissolved solids (organic and inorganic), dissolved gases (hydrogen sulfide, methane etc.) and micro-organisms (pathogen, bacteria and viruses); waste-water on the other hand can have much higher levels of impurities as earlier discussed because of the human organic waste added to water along with detergents, pesticides, and micro-organism.
But waste-water can be called as water of different form if the concentration of impurities is reduced, it can have applications similar to water.
It is the task in this course to have a level of waste-water treatment that the end product should not be harmful to the user, be it the water left after treatment or the sludge used for fertilization or disposed / deposited into the sea.
Read Also : Industrial Waste and their Impact on the Environment
Different Treatment Processes
Waste-water treatment processes normally uses physical processes initially and later chemical processes like precipitation. But if it is not sufficiently treated by the two processes, then the three can be used. Therefore, it should be known that waste-water treatment is divided into three main processes, herein given below.
Physical processes comprising screening or straining, sedimentation, flocculation and filtration. This should be designated as P (Physical process)
Chemical treatment using adsorption, coagulation, ion exchange, precipitation. This should be known as C (Chemical process)
Biological treatment processes with dispersed growth system (activated sludge, stabilization ponds); fixed film reactors (biological filters such as tricking filter). This is known as B (Biological process).
Assignment: Pick your pen and write out some of the discrete processes known to you but is/are not contained in the list above, classifying same as you do that.

Physical Treatment Processes
The main aim of physical treatment process in a water or waste-water treatment system is to protect the main treatment systems from possible damage or clogging. Various processes are used to remove large floating and suspended material.
Screening
This is the first operation in the physical treatment process. Micro strainer with specially woven stainless steel meshes size between 20 to 40 micrometer are usually used in water treatment plants.
The nature and quantity of screening collected depend upon the social, dietary, as well sewerage system of the city. It also depends on the screening bar spacing, which affect the loss of velocity head.
This head- loss indicates the performance of a screen. On an average its quantity ranges from .01 to .03 cu.m./1000 population per day.
Screening is unpleasant in nature with a strong odor, particularly in high temperatures. Their density is usually 1800-1900 kg/cu.m with a solid content of 15-30%.
Sample Problem
A bar screen is installed in a waste-water treatment plant receiving a daily peak flow of crude sewage of 50,000 cu.m. Estimate head-loss through the screen and also the gross area of the screen.
Solution
Maximum flow, Q= 50,000 cu.m. =.5788 cu.m/s.
Desired velocity through the screen, V,at ultimate flow= 0 .8m/s Net area of screen opening required, A= .5788/.8 = 0.72 m/s Since Q= V/A.
Using rectangular bars in the screen having 1 cm width, 1 m height and placed 5 cm clear spacing.
Number of bars required = .7235 * 100/5 = 14.46 Say 15 bars of 1 cm each.
Total area required for screen = 0.7235 + (15 * 10^-2) = 0.87 square meter.
Assuming that the inclination of the screen to the horizontal is at 60 degrees.
The gross area of screen would be;
= 0.87 * (square root of 3/ 2) = 1 square meter.
Velocity through the clear screen, v = 0.8m/s.
Velocity above the screen, u = 0.8 * 5/6 m/s = 0.67 m/s.
Using the equation of continuity;
Head- loss through the screen is usually given as;
– = 0.0729 (v^2- u^2).
– = 0.0729 (0.8^2 – 0.67^2).
– = 0.014 m.
If the screen openings are half plugged with screenings, leaves and debris, the velocity through the screen is doubled.
– v = 0.8 * 2 m/s.
– = 1.6 m/s.
Maximum head- loss = .0729 (1.6^2 – 0.67 ^2).
= 0.15m.
At this maximum head -loss, the screen is to be cleaned.
If you are not clear with the calculation try and read it over and over again before forging ahead. If at the end of the day you are still confused, consult your friends or superiors.
Comminution and Maceration
In situ maceration of floating and large suspended solids can be achieved by a comminutor or macerator in which the material is trapped between teeth mounted on a slotted rotating drum and a fixed comb.
Grit Removal
In most sewerage plant, considerable amount of sand and grit are transported to the waste-water treatment plant. These materials arise from roads and other paved areas.
These two should be removed earlier in order to prevent damage on the pumps and other mechanical equipment’s.
Sedimentation
Is the separation of solids based on difference between densities of solids and waste-water due to gravity?
Stoke‘slaw
Vs = (gd^2/18V) (Ss – 1)
Where Vs = discrete particle terminal velocity; g is acceleration due to gravity; d= diameter of the particle; V = kinematic viscosity of water
Ss = specific gravity of the particle
Practical consideration in settlement of the discrete suspension involves concept of ideal settling basin in which it is assumed that the following conditions exists.
Quiescent settlement in the settling zone
Uniform flow through the settling zone
Uniform solids concentrations entering the settling zone
Solids entering the sludge zone are not resuspended.
| Range of length/width ratio | 1.5- 7.5 |
| Range of length/ depth ratio | 5 – 25 |
| Bottom slope | 1% 7.5 – 10% ( from periphery to center) |
| Max. diameter | 30m |
| Inlet | Multiple pipes on the central inlet pipe with concentric width side with inlet baffle of diameter 15% of baffle boards of depth 0.5 tank diameter and extending m and 0.8 m in front 1m below surface of the pipe inlets and surface for scum Passover |
| Outlet | Overflow weir with V-notches to peripheral weir provided Provide uniform flow at low with V- notch. Scum baffle heads Scum baffles provide extending 0.3 m below ahead of weir for water surface provided wastewater installations ahead of effluent weir for waste-water installation |
| Peak velocity | Depends upon feed |
| Scraper arms velocity | 0.2 m.min Design features of waste-water sedimentation tank. |
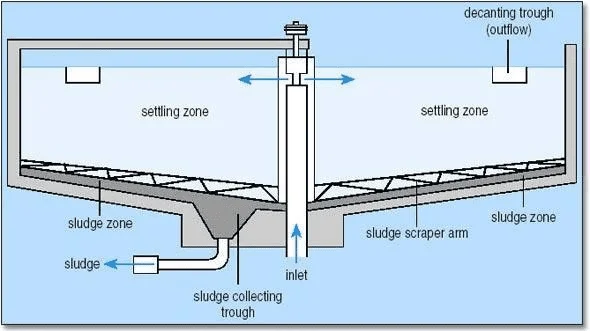
Typical sedimentation tanks include (a) rectangular horizontal flow tank circular, radial-flow tank (c) hopper-bottomed, upward flow.
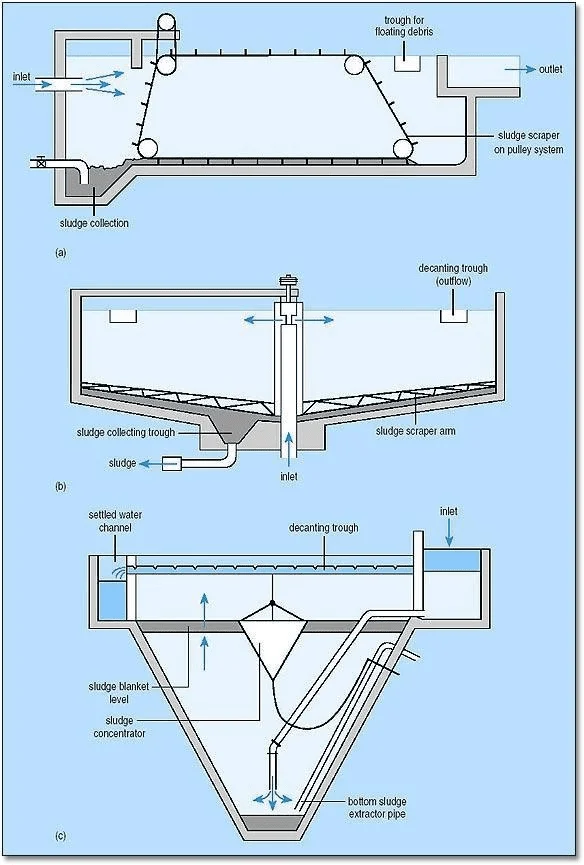
Diagrammatic Presentation of the Types
a. Inlet zone – at the central well, which has a round baffle plate, the flow is established in a uniform radial direction so that short- circuiting does not take place.
b. Settling zone– where settling is assumed to occur as the water flows towards the outlet.
c. Outlet zone – in which the flow converges up and over the decanting weirs.
d. Sludge zone – where settled material collects and is pumped out.
Flocculation
This process is used when the small suspended solids having low specific gravity and low settling velocity cannot be separated by sedimentation easily. In waste-water treatment, this usually occurs particularly with particles of less than 50 micrometer in size.
Chemical Treatment Processes
Chemical precipitation- This is the method of addition of chemicals to the waste-water, converting undesired soluble substances into an insoluble precipitate which can be removed easily and rapidly.
The undesired constituents generally found in waste-water are:
Suspended colloidal and supra colloidal solids and their associated organic matter. They should be removed in order to avoid damage to performance and reduction in efficiency.
Phosphorus
Toxic heavy metals and organics discharged from industrial plants and premises into the municipal waste-water treatment plant.
Coagulation
This is an important operation in removing colloidal solids. In waste- water treatment, chemical such as lime, ferric salts and commercial alum are used as coagulants. This process removes suspended solids (60- 80%), BOD (50-70%) phosphorus (over 90%) and heavy metal (over 80%).
Chemical clarification of waste-water can be combined with the activated carbon adsorption process to provide complete physical – chemical waste-water treatment.
The use of chemical clarification has restricted application in developing countries because of its constant requirement of consumable chemical and consequent higher running costs.
Biological Treatment Processes
In this method, use of microorganisms during the process is involved. Processes under this use mostly aerobic oxidation and include:
Filtration process
Trickling filter process
Oxidation pond application
These have been discussed in various units in the past.
Assignment: Find other methods that involve biological process not included in the list above. Do you still remember the above processes?
Advanced Treatment Processes
Advanced physical processes
Ammonia stripping
These are used in order to get better treatment efficiency. Some of the better known operations used in advanced treatment are ammonia stripping, distillation, foam, fractionation, and freezing.
It is also known as air stripping of ammonia. It is a modification of the aeration process used for the removal of gases dissolved in water.
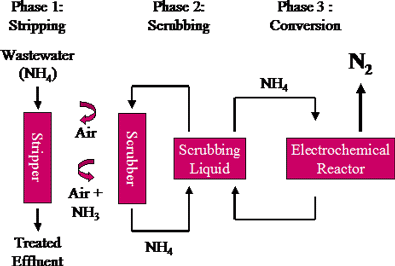
In the first phase, sodium hydroxide (NaOH) is added to the waste-water stream just before being pumped into the stripping column. This raises the water pH to the point where ammonium ions (NH 4+) are converted to ammonia gas.
Ammonia removal is enhanced by pumping the waste- water stream into the top of the column which is packed with high surface area packing material. Air is pumped into the bottom of the column. The ammonia carried by the air stream is then transferred to liquid in the scrub column.
At the core of AmmEL-HC is a patented electrochemical reactor that converts the ammonia dissolved in the scrubbing liquid to gaseous Nitrogen without producing nitrates or greenhouse gases.
Chemically inert and occupying an 80 per cent proportion of our atmosphere, gaseous nitrogen is the ideal end product for safe dispersal into the environment.
Testing of the AmmEL-HC system has demonstrated its capabilities. As shown below, a percentage removal ranging from 93% to 99% was achieved throughout the test.
Other advantages of AmmEL-HC include:
Improves performance of existing biological treatment plants by preventing ammonia toxicity in the activated sludge process.
Less expensive than conventional processes, including biological treatment.
Does not convert ammonia to nitrate and does not produce greenhouse gases.
Small footprint – can reduce overall cost of land for new biological treatment plants.
Can operate intermittently without start-up delays.
Fully automated – low maintenance.
Remotely monitored.
Distillation
This is a unit operation in which the components of a liquid solution are separated vaporization and condensation.
Foamfractionation
This is the separation of colloidal and suspended material by floatation and dissolved organic matter by adsorption.
Freezing
Waste-water is sprayed into a chamber operating under a vacuum. A portion of waste-water gets evaporated and cooling effect produces contaminant free ice crystals in the remaining liquid.
Gas phase separation
It is a promising method for removal of ammonia as gas. This method is based on the development of selective permeable gas-phase membranes.
Reverse osmosis
It is the process in which water is separated from dissolved salts in the solution by filtering through semi permeable membrane at a pressure than the osmotic pressure caused by the dissolved salts in waste-water.
Sorption
Conventional alum treatment for removal of phosphate increases the concentration of sulphate ions in the solution. To avoid this, sorption is developed. Sorption is a process of removing various forms of phosphate without increasing the concentration of sulphates.
Advanced chemical processes
Ion exchange- although both natural and synthetic ion exchange resins are available, synthetic resins are used more widely because of their durability. Some natural zeolites (resins) are also used for the removal of ammonia from waste-water.
Electro chemical treatment
Waste-water is mixed with seawater and is passed into a single cell containing carbon electrodes.
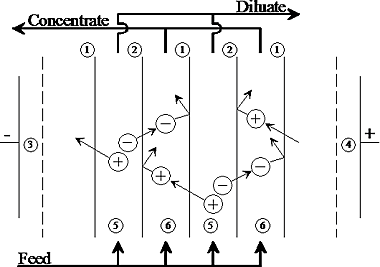
Electro dialysis
Ionic components of solutions are separated through the use of semi permeable ion-selective membranes.
If the electrical potential is applied between the two electrodes, it in turn causes a migration of cations toward the negative electrode and migration of anions towards the positive electrode.
Due to the alternate spacing of cation and anion permeable membranes, cells of concentrated and dilute salts are formed.
Principle of simple electrodialysis process – The diagram shows the membrane configuration with alternating cation-selective (1) anion- selective (2) membranes between two electrodes (3) and (4)), one at each end of the stack.
Oxidation and Reduction
Oxidation-Chemical oxidation is used to remove ammonia, to reduce the concentration of residual organics and to reduce bacterial and viral contents of waste-water. At present, one of the few processes for the removal of ammonical nitrogen, found operationally dependable, is chlorination.
Ammonia can be removed chemically by adding chlorine or hypochlorite to form monochloramine and dichloramine as intermediate products and nitrogen gas and hydrochloric acid as end products.
Problem associated with this method is the presence of various organic and inorganic compounds that will exert chlorine demand. Chemical oxidation of organic material in waste-water does this.
Reduction-Nitrates present in waste-water can be reduced electrolytically or by using strong reducing agents (e.g. ferrous oxide).
The reaction must usually be catalyzed while using reducing agents. The two step processes using different reducing agents and catalysts are limited by the availability of chemicals at low cost, and the fact that the treated effluent and waste sludge may contain toxic compounds derived from the chemicals used for catalyzing various reactions.
In conclusion, it shows the categorization of the processes involved which are mainly physical, biological and chemical processes. There are thus primary, secondary, tertiary or advanced forms of most of all the processes involved in the treatment as have been learnt in this unit and the preceding units.
The nature of impurities was done. Here we were made to understand that it is a collection of macro and micro materials that are contained in the waste-water which are of organic and inorganic origin, there are gases as well as micro-organisms also.
Read Also: Industrial Waste-water Treatment Trickle Filters
Different treatment processes were equally learnt. Here, we were made to understand that there are three major different methods, viz:
Physical processes comprising screening or straining, sedimentation, flocculation and filtration. This is be designated as P (Physical process).
Chemical treatment using adsorption, coagulation, ion exchange, precipitation. This is known as C (Chemical process).
Biological treatment processes with dispersed growth system (activated sludge, stabilization ponds); fixed film reactors (biological filters such as tricking filter). This is known as B (Biological process).
Read Also: Appearance, Features and Farming Guide of Pangasius Fish

