Apart from microbial degradation, pesticides are also converted in the environment through many different mechanisms. Physical and chemical agents play significant roles in the transformation of insecticide, herbicide, and fungicide molecules to various degradation products.
Transformation mechanisms include oxidation, hydrolysis, reduction, hydration, conjugation, isomerization, and cyclization. Resultant products are usually less bioactive than the parent pesticide molecule, but numerous cases have been documented of metabolites with greater bioactivity.
The physical and chemical properties of the degradation products are also different from those of the parent compound, and their fate and significance in the environment also are altered with the structural changes.
The concept of “environmental activation” describes the transformation of a pesticide to a degradation product that is of significance in the environment as a result of its environmental toxicology or chemistry (Coats, 1991).
Non-Enzymatic Conversion
Processes involved in the conversion of pesticides outside the biological processes previously discussed are termed non-enzymatic conversion. They are mainly physical and chemical processes.
Read Also : Soil Herbicide Interaction and Herbicide Efficacy
A combination of factors usually influence the breakdown of a pesticides over a time period of hours, days, weeks, months, or years, and through many of the situations enumerated above.
Factors that influence the relative importance of the various transformation agentsdepend to a great extent on the chemical’s use pattern, physical properties, and chemical structure. The two primary physical agents involved in the degradation process are light and heat.
Photolysis of pesticide residues is extremely significant on vegetation, on the soil surface, in water, and in the atmosphere (Matsumura, 1985).
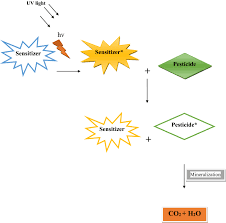
Thermal decomposition of the chemicals often occurs concomitantly with the photodegradative reactions; solar radiation, therefore, is directly responsible for the decomposition in two ways, through photolysis and thermal decomposition.
Cold, especially freezing, temperatures can also contribute occasionally to pesticide degradation if certain formulations are allowed to freeze, and the pesticide is forced out of solution, suspension, or microencapsulation, making it more susceptible to degradative forces before and after application.
Chemical degradation occurs as a result of the various reactive agents in the formulations, tank mixes, and in the environment. Water is responsible for considerable breakdown of pesticides in solution, especially in conjunction with pH extremes.
Even slight variance from a neutral pH can elicit rapid decomposition of pH-sensitive compounds. Molecular oxygen and its several more reactive forms (e.g., ozone, superoxide, peroxides) are capable of reacting with many chemicals to generate oxidation products. In all but the most oxygen-poor environments, oxidative transformations are frequently the most common degradation pathways observed.
Other chemical oxidations, as well as reductions, can progress in the presence of certain inorganic redox reagents (Manahan, 1990). These reactive species of metals function as highly effective catalysts which effect pesticide transformations in soils and in aquatic and marine environments.
Hydrolysis Reaction
For many pesticide molecules, hydrolysis is a primary route of degradation. Many types of esters are hydrolytically cleaved, yielding two fragments with little or no pesticidal activity.
Hydrolysis of esters can occur by chemical means; even mildly alkaline solutions can cause hydrolytic decomposition of some esters (organophosphorus, carbamate, pyrethroid), while acidcatalyzed hydrolysis typically is induced only by strongly acidic solutions.
Chemical hydrolysis of chlorides and bromides can occur, yielding a hydroxylated product. Hydrolysis of an epoxide, by an epoxide hydratase, produces a diol (Sipes andGandolfi, 1986). Oximecarbamates can be hydrolyzed to an oxime and a carbamic acid (Kuhr, 1975).
Oxidation
The mechanisms of oxidation vary. Physical and chemical oxidations involve molecular oxygen or more reactive species including various acids, peroxides, orsinglet oxygen, and are often enhanced by light, heat, or oxidized metals in soil or water.
Ring hydroxylation occurs on aromatic rings in pesticides of several types, as well as polycyclic aromatic hydrocarbons (PAH). It is easily accomplished on single or multiple-ring molecules that are unsubstituted or activated.
Substitution with bulky or multiple groups can sterically inhibit the reaction, as can the presence of deactivating substituents such as halogens.Any methylene group in an aliphatic side-chain is susceptible to oxidation.
Carbofuran undergoes hydroxylation of a methylene in the furan ring to form 7-hydroxycarbofuran, followed by further oxidation to 7- ketocarbofuran. Terminal methyl groups are also susceptible to hydroxylation.
The conversion of alkenes to epoxides does not radically alter the polarity of the substrate, nor the biological activity in some cases, e.g., aldrinls epoxidation to dieldrin. Epoxide formation may be, in fact, critical to bioactivity, e.g., precocenes require activation to be cytotoxic to the corpora allatum of insects to inhibit the production of juvenile hormone (Bowers, 1982).
Epoxides can be hydrolyzed to form diols; the net effect of the arene oxide formation followed by hydrolysis, is ring hydroxylation.
Reductions
Pesticides undergo reduction reactions in reducing environments, characterized by low oxygen concentrations, low pH’s, and anaerobic microorganisms.
Examples of situations in which pesticides are reduced include stagnant or eutrophic ponds and lakes, bogs, flooded rice fields, the rumens of ruminant livestock, and lower intestines of some species, including man. Reduction of a nitro group has been observed in flooded soil.
The fungicide pentachloronitrobenzene has also been shown to undergo reduction to an amino analog.
Read Also : Roles of Producers and Corporations in Waste Management
4Photo-degradation is the breakdown of pesticides by sunlight. All pesticides are susceptible to photo-degradation to some extent. The rate of breakdown is influenced by the intensity and spectrum of sunlight, length of exposure, and the properties of the pesticide.
Pesticides applied to foliage are more exposed to sunlight than pesticides that are incorporated into the soil. Photo-degradation can destroy pesticides on foliage, on the soil surface, and even in the air.
Pesticides may break down faster inside plastic-covered greenhouses than inside glass greenhouses, since glass filters out much of the ultraviolet light that degrades pesticides.
Factors that influence pesticide photo-degradation include the intensity of the sunlight, properties of the application site, the application method, and the properties of the pesticide.

