Industries represent human activities, and they are the most veritable sources of generation of pollutants into the environment. Efforts geared towards the control of pollution at the source of generation are very important in environmental Health. In modern day practice, the use of equipment’s is more effective and more assuring. In this unit we are going to examine the workings of the different types of pollution control equipment we have.
Pollution Control
Pollution control is the process of reducing or eliminating the release of pollutants into the environment. It is regulated by various environmental agencies which establish pollutant discharge limits for air, water, and land.
Control of environmental pollution at source is very good attempt resulting in reduction of environmental pollution which not only effect human beings but also the other wonderful creatures living in this beautiful world. Method of controlling air and water pollution should be given thrust as everybody will get affected by the same.
Pollution control board should take necessary action at their end with the full cooperation of the public at large. Pollution has become the biggest problem in today‘s world. No place is left over on earth without pollution and pollutants.
Air pollution control strategies can be divided into two categories, the control of particulate emission and the control of gaseous emissions. There are many kinds of equipment which can be used to reduce particulate emissions.
Read Also: Definition and Concept of Pollution Abatement and Control
Physical separation of the particulate from the air using settling chambers, cyclone collectors, impingers, wet scrubbers, electrostatic precipitators, and filtration devices, are all processes that are typically employed.
Control Devices/ Methods in Industries
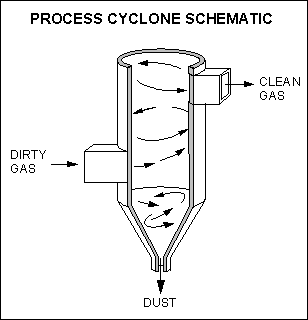
1. Cyclones
Cyclones operate to collect relatively large size particulate matter from a gaseous stream through the use of centrifugal forces.
Dust laden gas is made to rotate in a decreasing diameter pathway forcing solids to the outer edge of the gas stream for deposition into the bottom of the cyclone. 90% efficiency in particle sizes of 10 microns or greater are possible.
Performance & Collection Efficiency
Linear increases with particle density, gas stream velocity, and rotational passes
Linear decrease with fluid viscosity Exponential increase with particle diameter. Limitations / Advantages / Problems Reduces internal access needs
Optimal flow rate difficult to adjust Prone to internal erosion / corrosion Operation at elevated temperatures possible Low efficiency for small diameter material
Hopper recirculation / flow distribution problems High energy costs for volumetric flow requirements Dew point agglomeration, bridging, and plugging
Few moving parts, few mechanical / electrical ignition sources.
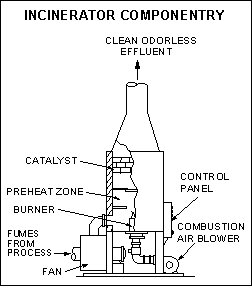
2. Incinerators
Incineration involves the high efficiency combustion of certain solid, liquid, or gaseous wastes. The reactions may be self-sustaining based on the combustibility of the waste or require the addition of fuels.
Read Also: The Economic Impact of Pollution
They may be batch operations or continuous as with flares used to burn off methane from landfills; and, they may incorporate secondary control methods and operate at efficiency levels of 99.99%, as with hazardous waste incinerators.
Combustion temperatures, contact time, and mass transfer are the major parameters affecting incineration performance.
Performance & Efficiency Parameters
t = V/Q t = residence time where; V = incinerator volume
Q = gas volumetric flow rate at combustion conditions Increased residence times mean increased performance
Hydrocarbon incineration rate = d [HC] = -k [HC]
Where, [HC] = concentration of hydrocarbon
-k = reaction rate constant Increased residence times mean increased performance
Increased waste stream concentrations mean increased percentage rates of incineration
Limitations / Advantages / Problems High destruction efficiencies possible Variations in fuel content of waste
Transition among wastes require significant control changes Good for gases, liquids, and solids
High cost of supplementary fuel
High temperatures require good thermal loss control
Hot surfaces, flashback, and explosive conditions.
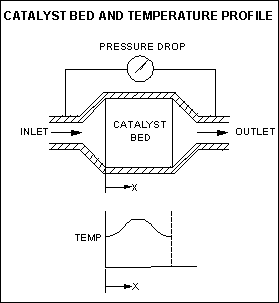
3. Catalytic Reactors
Catalytic reactors can perform similar thermal destruction functions as incinerators, but for selected waste gases only.
They incorporate beds of solid catalytic material that the unwanted gases pass through typically for oxidation or reduction purposes, and have the advantages of lowering the thermal energy requirements and allow small, short-term fluctuations in stoichiometry.
Efficiency kevels of 99.99% are possible with reduced energy costs. Increasing pressure drops across the catalyst bed increase energy / operating costs.
Performance & Efficiency Parameters
Pressure drop = (L) (uf) (V²)
| where; | L | = bed thickness |
| V | = velocity | |
| uf | = fluid viscosity | |
| A | = cross-sectional area |
Limitations/ Advantages/ Problems Supplementary fuel savings
Short-circuiting of flow through bed Excessive oxidation and thermal failure Breakthrough of emissions as failure mode Abrasion and thermal shock of catalyst Poisoning of catalyst and drop in performance
Thick beds cause high pressure drops and increased energy costs.
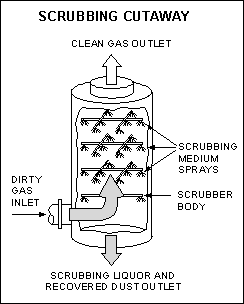
4. Absorption & Wet Scrubbing Equipment
The goal in absorption and wet scrubbing equipment is the removal of gases and particulate matter from an exhaust stream by causing the gaseous contamination to become dissolved into the liquid stream and the solids to be entrained in the liquid.
The rate of gas transfer into the liquid is dependent upon the solubility, mass transfer mechanism, and equilibrium concentration of the gas in solution. Gas collection efficiencies in the range of 99% are possible.
The rate of particulate matter collection at constant pressure drops is inversely proportional to the aerodynamic mean diameter of the particulate matter and scrubber droplets.
Where, Hk) is Henry’s constant
[Cgas] is the concentration in the gas stream [Cliquid] is the concentration in the liquid stream
Performance & Efficiency Parameters
For gas collection, the maximum equilibrium concentration in solution is described by Henry’s law:
[Cgas] = (Hk) [Cliquid]
Limitations / Advantages / Problems High pressure drops required internal plugging, corrosion, and erosion
Increased need for internal inspection Formation / precipitation of solids few internal moving parts
Reduced opportunity for gas ignition
Gas and liquid chemistry control important
Increased relative velocity between scrubbing the fluid and gas stream, increases efficiency for solids.
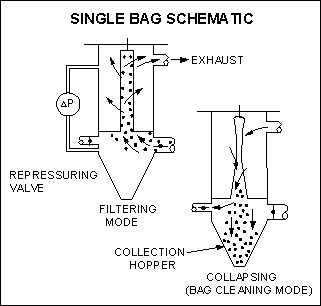
5. Bag houses
Bag houses utilize sieving, impaction, agglomeration, and electrostatic filtration principles to remove solids from a gaseous exhaust stream.
Bag houses maximize the filtration area by configuring the fabric filter media into a series of long small-diameter fabric tubes referred to as bags.
They are tightly packed into a housing wherein the dust laden air moves across the bag fabric thereby removing it from the gas stream and building up a filter cake which further enhances air cleaning.
The filter cake is removed to hoppers by various shaking means. The operating pressure drop across the bags is described by:
Performance& Efficiency Parameters
Pressure drop = dP = SeV + KCV2t
| where, | Se | = drag coefficient |
| V | = velocity | |
| K | = filter cake coefficient | |
| C | = inlet dust concentration | |
| t | = Collection running time |
Limitations/Advantages/ Problems High collection efficiencies
Internal condensation / corrosion Over-temperature limitations
Need for internal inspection / access Possible to have variable flow rates
Plugging / short-circuiting / break-through/ collection media fouling Accumulation of flammable gases/ dusts and ignition sources Unexpected bag failure due to changes in operating parameters.
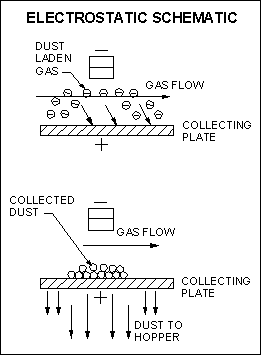
6. Electrostatic Precipitators
This control device utilizes gaseous ions to charge particles which are then moved through an electric field to be deposited onto charged collection plates. Collected particulate material is then removed by rapping or washing of the plates. To produce the free ions and electric field, high internal voltages are required.
Since the collection process does not rely on mechanical processes such as sieving or impaction, but rather electrostatic forces, the internal gas passages within a precipitator are relatively open with small pressure drops and lower energy costs to move the gas stream. High collection efficiencies are possible, but collecting efficiency may drastically change with changes in operating parameters.
Performance& EfficiencyParameters
Collection Eff. % = 1 – e -WA/Q
C = proportionality constant n = gas viscosity and drift vel. = EoEpaC W (pi) n
Limitations/Advantages/Problems
Large installation space required
High efficiencies for small particles possible Low pressure drops and air moving costs High potential for ignition sources
Re-entrainment, spark-over, back corona problems High temperature operation possible
Susceptible to changes in moisture and resistivity
7. Adsorption
The process of adsorption involves the molecular attraction of gas phase materials onto the surface of certain solids. This attraction may be chemical or physical in nature and is predominantly a surface effect.
Certain materials like activated carbon charcoal possess the large internal surface area and the presence of physical attraction forces to adsorb large quantities of certain gases within their structure. The rate of adsorption is affected by the temperature, concentration, atmospheric pressure, and molecular structure of the gas.
Performance& Efficiency Parameters
The following figure shows typical trends for adsorption. Limitations / Advantages / Problems
Can recover contaminant for reuse
May require multiple units; one in service, one in recycle mode
Few internal parts, controls, and alternating cycler required Potential for step-function change in efficiency
Normal operation at ambient temperature Flammable hydrocarbons
Chemical mixture problems.
Other Methods
The method of controlling air pollution at source is complex. It can be controlled by proper use of raw materials, site locations for industries and using the modern technologies to reduce emissions.
There is a mass transfer between soluble gas component and a solvent liquid HCl, HF and others can be removed by dissolving them in water. SO2 can be absorbed in alkaline water. The attraction forces between atoms, molecules and ions which hold a solid together fail at the surface of solids.
Hence they cannot hold other materials such as gases and liquids, the absorption capacity of solid increases with its porosity. Activated carbon, silica gets and earth are some of the important absorbents. The absorbents placed in suitable containers capture gaseous or liquid molecules passing through them.
Vapors are usually controlled by condensation and are a suitable measure to combat the emissions of hydrocarbons and organic compounds having low vapor pressure. Gaseous pollutants can be controlled by chemical reactions with other elements. Setting chambers are used to collect the heavy particles from a gas stream.
The particles settle out due to gravity action. Electrical precipitators containing charged plates in the air or gas stream settle the particles with opposite charge. The use of natural gas instead of coal can avoid sulphur oxide and fly ash emission from power plants. LPG or CNG can be used instead of petrol and diesel in automobiles.
Settling chambers use gravity separation to reduce particulate emissions. The air stream is directed through a settling chamber, which is relatively long and has a large cross section, causing the velocity of the air stream to be greatly decreased and allowing sufficient time for the settling of solid particles.
Another means of controlling both particulate and gaseous air pollutant emission can be accomplished by modifying the process which generates these pollutants. For example, modifications to process equipment or raw materials can provide effective source reduction. Also, employing fuel cleaning methods such as desulfurisation and increasing fuel-burning efficiency can lessen air emissions.
Water pollution control methods can be subdivided into physical, chemical, and biological treatment systems. Most treatment systems use combinations of any of these three technologies. Additionally, water conservation is a beneficial means to reduce the volume of wastewater generated.
Physical treatment systems are processes that rely on physical forces to aid in the removal of pollutants. Physical processes which find frequent use in water pollution control include screening, filtration, sedimentation, and flotation. Screening and filtration are similar methods used to separate coarse solids from water.
Suspended particles are also removed from water with the use of sedimentation processes. Just as in air pollution control, sedimentation devices utilize gravity to remove the heavier particles from the water stream.
The wide array of sedimentation basins in use slow down the water velocity in the unit to allow time for the particles to drop to the bottom. Likewise, flotation uses differences in particle densities, which in this case are lower than water, to effect removal.
Fine gas bubbles are often introduced to Equipment for the complete recovery and control of air, acids, and oxide emissions.
Chemical treatment systems in water pollution control are those processes which utilize chemical reactions to remove water pollutants or to form other, less toxic, compounds. Typical chemical treatment processes are chemical precipitation, adsorption, and disinfection reactions.
Chemical precipitation processes utilize the addition of chemicals to the water in order to bring about the precipitation of dissolved solids.
The solid is then removed by a physical process such as sedimentation or filtration. Chemical precipitation processes are often used for the removal of heavy metals and phosphorus from water streams.
Adsorption processes are used to separate soluble substances from the water stream. Like air pollution adsorption processes, activated carbon is the most widely used adsorbent.
Water may be passed through beds of Granulated Activated Carbon (GAC), or Powdered Activated Carbon (PAC) may be added in order to facilitate the removal of dissolved pollutants.
Disinfection processes selectively destroy disease- causing organisms such as bacteria and viruses. Typical disinfection agents include chlorine, ozone, and ultraviolet radiation.
Biological water pollution control methods are those which utilize biological activity to remove pollutants from water streams.
These methods are used for the control of biodegradable organic chemicals, as well as nutrients such as nitrogen and phosphorus. In these systems, microorganisms consisting mainly of bacteria convert carbonaceous matter as well as cell tissue into gas.
There are two main groups of microorganisms which are used in biological treatment, aerobic and anaerobic microorganisms. Each requires unique environmental conditions to do its job. Aerobic processes occur in the absence of oxygen.
Both processes may be utilized whether the microorganisms exist in a suspension or are attached to a surface. These processes are termed suspended growth and fixed film processes, respectively.
Read Also: Institutional Arrangement for Pollution Control
Solid pollution control methods that are typically used include land filling, composting, and incineration. Sanitary landfills are operated by spreading the solid waste in compact layers separated by a thin layer of soil.
Aerobic and anaerobic microorganisms help break down the biodegradable substances in the landfill and produce carbon dioxide and methane gas, which is typically vented to the surface. Landfills also generate a strong wastewater called Leachate that must be collected and treated to avoid groundwater contamination.
Composting of solid wastes is the microbiological biodegradation of organic matter under either aerobic or anaerobic conditions. This process is most applicable for readily biodegradable solids such as sewage sludge, paper, food waste, and household garbage, including garden waste and organic matter. This process can be carried out in static pile, agitated beds, or a variety of reactors.
In conclusion, there are many different types of methods of handling pollution in the industry. Whatever method is chosen the important thing is to ensure that it works and is able to create a barrier between pollutants and the environment or human health on the other hand.
There are different methods of handling pollution in the industry. These methods include; bag houses, cyclone, catalytic reactors, incinerators, absorption, and the use of scrubbers.
Other methods include physical techniques of water treatment involving; screening, sedimentation, aeration, filtration, etc. Landfills and composting are also used in the control of solid wastes.

